The Risks of Ochratoxin A in Food
Ochratoxin A, also known as OTA, is a fungal toxin produced by some fungal species. It contaminates humans through food, and is found in dozens of them in almost every country in the world. There are multiple health risks of ochratoxin A in food, such as the formation of kidney tumors and liver damage.
Although we have known for decades about the risk associated with the presence of this toxin in the diet, its interest has recently been renewed due to the measures taken against it. In the same way, recent reports and studies have corroborated the suspicions of health complications resulting from its intake. Let’s take a look at everything you need to know about the risks of ochratoxin A in food.
What is ochratoxin A?
Ochratoxin A is a fungal toxin, a mycotoxin, produced by certain species of fungi in the genera Aspergillus and Penicillium. The main species that generate this mycotoxin are P. verrucosum, A. carbonarius, A. niger and A. ochraceus. Several types of ochratoxins have been identified naturally, but type A is the most common and, at the same time, the most dangerous of all.
The fungi that produce the toxin must grow under specific conditions in order to generate it as a byproduct of their metabolism. Specialists state that the conditions for the two genders are the following:
- Temperature: 31°C for Aspergillus and 20°C for Penicillium.
- Minimum water activity: 0.8 for Aspergillus and 0.86 for Penicillium.
Therefore, the toxin isn’t produced in all contexts; and even the fungi themselves require very specific conditions in order to grow. If anything, ochratoxins of any kind are found mainly in the cereals of Northern Europe and Africa.

According to experts, contamination occurs as a result of poor storage of basic products. Suboptimal farming practices during food drying are also a catalyst for contamination. Here’s a list of the main foods this substance is usually found in:
- Smoked dried fish
- Dry beans
- Nuts
- Pepper
- Chickpeas
- Coffee grains
- Wheat, flour and bran
- Corn, barley and rice
- Grapes and products derived from grapes (such as wine)
- Some citrus fruits (apples, pears, peaches, figs and others)
- Meat products and cheese
Since OTA is chemically very stable, most food processing measures fail to substantially reduce its presence. In low concentrations, OTA doesn’t usually cause complications, so that the risks appear in moderate or high concentrations.
Risks of ochratoxin A in food
In the early 1970s, the first reports of the possible relationship between ochratoxin A in food and health problems appeared. Complications were reported in European countries such as Croatia, Bulgaria, Serbia, Romania, and Slovenia; also in African countries such as South Africa, the Congo, Tunisia, Egypt and Morocco.
Most of the studies have been carried out on animals. For example, it is known to cause nephropathy in pigs; a relatively common problem in central and northern Europe.
Similarly, there is evidence that mycotoxin exposure causes kidney tumors in rodents. It has also been found that in the latter it causes immunotoxicity. In the case of humans, the risks of ochratoxin A in food are as follows.
1. Nephropathy
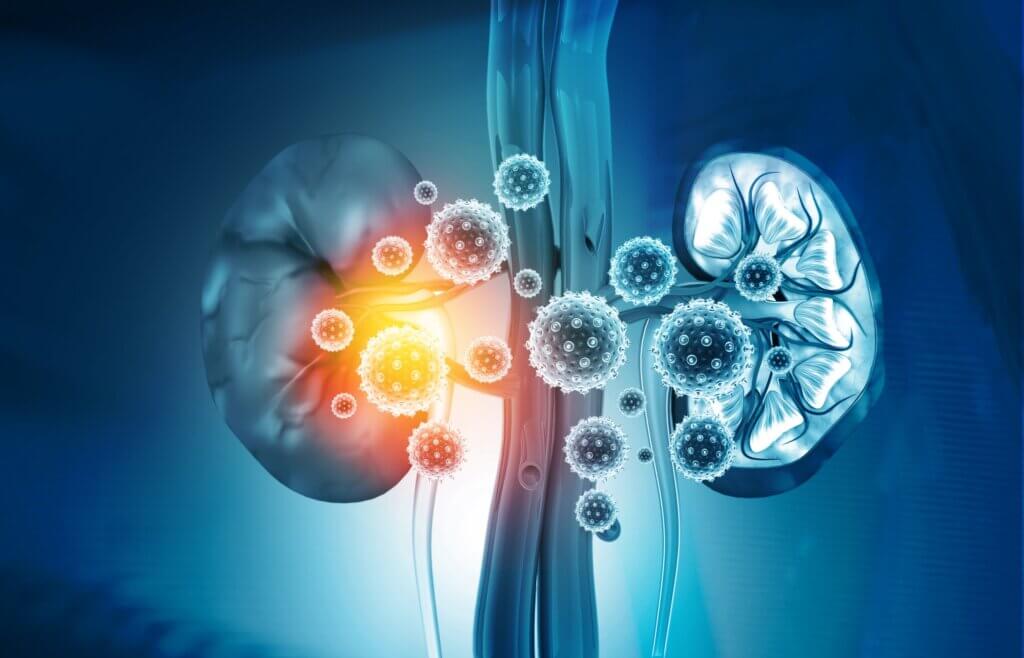
Various investigators have associated this mycotoxin with Balkan endemic nephropathy. Cases of renal failure have been reported after inhalation, so nephropathy is the main complication associated with the toxin.
2. Liver toxicity
The life cycle of this mycotoxin in the human body is 35 days immediately after oral ingestion. This is because it is reabsorbed during the enterohepatic circulation. Considering that people ingest it regularly, it’s very likely that, for long periods of time, the body has traces of ochratoxin A. Cases of liver toxicity may occur as a consequence.
3. Carcinogenesis
There is evidence that high intake of this mycotoxin causes tumors in the urinary tract. Similarly, experts warn that acromegaly in proximal and distal tubular epithelial cells is also a reported complication.
These are the main risks of ochratoxin A in food. Due to this, various institutions, organizations, and governments around the world have taken measures to regulate their presence. Most complications arise after chronic exposure, especially in regions that have the perfect conditions for the mycotoxin to be generated as a by-product.
There’s no consensus on the recommended daily or weekly amount that a person can ingest to avoid sequelae. A maximum of 3 nanograms/kilogram of body weight/day has been suggested, although recommendations differ widely around the world. In any case, the risks are well established and in many contexts are underestimated by both producers and consumers.
Ochratoxin A, also known as OTA, is a fungal toxin produced by some fungal species. It contaminates humans through food, and is found in dozens of them in almost every country in the world. There are multiple health risks of ochratoxin A in food, such as the formation of kidney tumors and liver damage.
Although we have known for decades about the risk associated with the presence of this toxin in the diet, its interest has recently been renewed due to the measures taken against it. In the same way, recent reports and studies have corroborated the suspicions of health complications resulting from its intake. Let’s take a look at everything you need to know about the risks of ochratoxin A in food.
What is ochratoxin A?
Ochratoxin A is a fungal toxin, a mycotoxin, produced by certain species of fungi in the genera Aspergillus and Penicillium. The main species that generate this mycotoxin are P. verrucosum, A. carbonarius, A. niger and A. ochraceus. Several types of ochratoxins have been identified naturally, but type A is the most common and, at the same time, the most dangerous of all.
The fungi that produce the toxin must grow under specific conditions in order to generate it as a byproduct of their metabolism. Specialists state that the conditions for the two genders are the following:
- Temperature: 31°C for Aspergillus and 20°C for Penicillium.
- Minimum water activity: 0.8 for Aspergillus and 0.86 for Penicillium.
Therefore, the toxin isn’t produced in all contexts; and even the fungi themselves require very specific conditions in order to grow. If anything, ochratoxins of any kind are found mainly in the cereals of Northern Europe and Africa.

According to experts, contamination occurs as a result of poor storage of basic products. Suboptimal farming practices during food drying are also a catalyst for contamination. Here’s a list of the main foods this substance is usually found in:
- Smoked dried fish
- Dry beans
- Nuts
- Pepper
- Chickpeas
- Coffee grains
- Wheat, flour and bran
- Corn, barley and rice
- Grapes and products derived from grapes (such as wine)
- Some citrus fruits (apples, pears, peaches, figs and others)
- Meat products and cheese
Since OTA is chemically very stable, most food processing measures fail to substantially reduce its presence. In low concentrations, OTA doesn’t usually cause complications, so that the risks appear in moderate or high concentrations.
Risks of ochratoxin A in food
In the early 1970s, the first reports of the possible relationship between ochratoxin A in food and health problems appeared. Complications were reported in European countries such as Croatia, Bulgaria, Serbia, Romania, and Slovenia; also in African countries such as South Africa, the Congo, Tunisia, Egypt and Morocco.
Most of the studies have been carried out on animals. For example, it is known to cause nephropathy in pigs; a relatively common problem in central and northern Europe.
Similarly, there is evidence that mycotoxin exposure causes kidney tumors in rodents. It has also been found that in the latter it causes immunotoxicity. In the case of humans, the risks of ochratoxin A in food are as follows.
1. Nephropathy

Various investigators have associated this mycotoxin with Balkan endemic nephropathy. Cases of renal failure have been reported after inhalation, so nephropathy is the main complication associated with the toxin.
2. Liver toxicity
The life cycle of this mycotoxin in the human body is 35 days immediately after oral ingestion. This is because it is reabsorbed during the enterohepatic circulation. Considering that people ingest it regularly, it’s very likely that, for long periods of time, the body has traces of ochratoxin A. Cases of liver toxicity may occur as a consequence.
3. Carcinogenesis
There is evidence that high intake of this mycotoxin causes tumors in the urinary tract. Similarly, experts warn that acromegaly in proximal and distal tubular epithelial cells is also a reported complication.
These are the main risks of ochratoxin A in food. Due to this, various institutions, organizations, and governments around the world have taken measures to regulate their presence. Most complications arise after chronic exposure, especially in regions that have the perfect conditions for the mycotoxin to be generated as a by-product.
There’s no consensus on the recommended daily or weekly amount that a person can ingest to avoid sequelae. A maximum of 3 nanograms/kilogram of body weight/day has been suggested, although recommendations differ widely around the world. In any case, the risks are well established and in many contexts are underestimated by both producers and consumers.
- Bondy GS, Pestka JJ. Immunomodulation by fungal toxins. J Toxicol Environ Health B Crit Rev. 2000 Apr-Jun;3(2):109-43.
- Bui-Klimke TR, Wu F. Ochratoxin A and human health risk: a review of the evidence. Crit Rev Food Sci Nutr. 2015;55(13):1860-9.
- Di Paolo N, Guarnieri A, Loi F, Sacchi G, Mangiarotti AM, Di Paolo M. Acute renal failure from inhalation of mycotoxins. Nephron. 1993;64(4):621-5.
- Kuiper-Goodman T, Hilts C, Billiard SM, Kiparissis Y, Richard ID, Hayward S. Health risk assessment of ochratoxin A for all age-sex strata in a market economy. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010 Feb;27(2):212-40.
- Krogh P, Elling F, Friis C, Hald B, Larsen AE, Lillehøj EB, Madsen A, Mortensen HP, Rasmussen F, Ravnskov U. Porcine nephropathy induced by long-term ingestion of ochratoxin A. Vet Pathol. 1979 Jul;16(4):466-75.
- Mantle P, Kulinskaya E, Nestler S. Renal tumourigenesis in male rats in response to chronic dietary ochratoxin A. Food Addit Contam. 2005;22 Suppl 1:58-64.
- Pfohl-Leszkowicz A, Tozlovanu M, Manderville R, Peraica M, Castegnaro M, Stefanovic V. New molecular and field evidences for the implication of mycotoxins but not aristolochic acid in human nephropathy and urinary tract tumor. Mol Nutr Food Res. 2007 Sep;51(9):1131-46.
- Reddy L, Bhoola K. Ochratoxins-food contaminants: impact on human health. Toxins (Basel). 2010 Apr;2(4):771-9.
- Simon, P. Ochratoxin and kidney disease in the human. Journal of Toxicology: Toxin Reviews. 1996; 15(3): 239-249.
Este texto se ofrece únicamente con propósitos informativos y no reemplaza la consulta con un profesional. Ante dudas, consulta a tu especialista.







